Dictum Health Receives FDA 510K Clearance for Portable Spirometry for In-Clinic Use and Remote Patient Monitoring
Expanding Telehealth to Include the Most At-Risk Patients
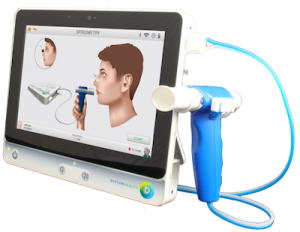
Oakland, Ca, August 8, 2017 – Dictum Health, Inc., innovators in telehealth solutions for acute, post-acute, and remote patient monitoring, is excited to announce that the company received FDA 510k clearance for spirometry, completing the cardiopulmonary capability of the IDM100 Medical Tablet. The system provides the most medically comprehensive telehealth solution in the market for clinical care anytime, anywhere.
Intended for use with pediatrics and adults under supervision of a healthcare professional, the fully integrated diagnostic spirometer provides interpretation for immediate diagnostic assessment. The IDM100 Medical Tablet delivers the same clinical accuracy as an in-hospital exam, improving patient outcomes and reducing the risk of readmissions.
“Using the IDM100 Medical Tablet with the spirometry capability enables me to diagnose and assess pediatric patients’ pulmonary conditions, whether in my office or from a remote location,” said Dr. Alan Greene, pediatrician and founder of DrGreene.com.
Clinicians can connect to a specialist for further assessment, or remotely monitor their most at-risk patients who are being treated for diseases such as asthma, COPD, heart failure, hypertension, and children with asthma. Physicians use Virtual Exam Room (VER) to conduct comprehensive, remote patient examinations with secure video conferencing and simultaneous streaming of vital signs, cardiopulmonary diagnostics information, and medical images.
“Our telehealth system provides real-time, clinically accurate vital signs, and now with the addition of spirometry, a complete cardiopulmonary assessment for remote patients with complex medical conditions,” said Elizabeth Keate, Dictum Health’s Vice President of Product Management. “What makes portable spirometry unique is providing the same clinical accuracy whether the patient is in a physician office, a remote care center, or recovering in their home.”
About Dictum Health
Dictum Health Inc., with offices in Oakland, Calif., Portsmouth, N.H. and Dubai, UAE, is an ISO 13485 Medical Device company offering next-generation, mobile and cloud-based telehealth solutions that connect physicians, patients and other members of the care team. Built upon a HIPAA compliant, cyber-secure platform, the products are FDA 510k cleared and provide a Virtual Exam Room (VER) with simultaneous real-time health-data streaming and vital sign and cardio pulmonary monitoring while delivering the same clinical accuracy as an inhospital exam for clinical care anytime, anywhere. For more information visit www.dictumhealth.com.
Contact:
Taryn Smith, Manager Marketing
Tel: 510.990.3153
Email: tsmith@dictumhealth.com
Source: Dictum Health, Inc.

